APPENDIX 7 – WORK INSTRUCTIONS
A nationwide tuberculin survey is being carried out, in order to assess the current epidemiological situation of tuberculosis in different parts of the country by computing Annual risk of tuberculosis infection (ARTI). The objective of the survey is to estimate the prevalence of tuberculosis infection among 1-9 year old children and to compute the ARTI from the estimated prevalence. For the purpose of the survey, the country has been divided into four geographical zones i.e., north, south, east and west. In each zone, the survey shall be carried out in six districts selected by stratified random sampling method.
STUDY POPULATION
The survey shall be carried out among 1-9 year old children residing in statistically selected rural and urban areas of the identified districts.
SELECTION OF CLUSTERS
In each zone, the survey shall be conducted in six districts. For purpose of the survey, a village is considered as a rural cluster and in the urban areas, a ward is considered as an urban cluster. The appropriate number of clusters shall be selected using PPS (population proportion to size) method. In each cluster, 85 eligible children shall be registered (See under Registration on Page No.3)
STAFF
A field team shall consist of the following members :
1. |
|
: | 2 |
2. |
|
: | 2 |
3. |
|
: | 2 |
4. |
|
: | 2 |
5. |
|
: | 2 |
The District Health Authorities shall be requested to depute 3 personnel of the level of health assistants in order to provide local support, 1 each for planning, testing and the reading teams.
The testing team consists of one tuberculin tester and one tester-secretary. The reading team shall consist of one reader and one reader-secretary.
The entire field work shall be carried out under the constant supervision of senior field staff from NTI/other collaborating agency. A designated Medical Officer from NTI/other collaborating agency shall be in-charge of the survey in each zone. The Epidemiologist, NTI, shall be overall in-charge of the survey in the entire country.
FIELD PLANNING
| i) | The state health authorities (State TB Officer) and the district health authorities including Deputy Commissioner shall be informed in advance about the purpose of the survey and the time period when it has to be conducted. Their necessary co-operation will be sought by NTI/ collaborating agency much in advance of the fieldwork. |
|
| ii) | The team will camp at the accommodation arranged by the district health office/ rented accommodation at district headquarters for the period of study in that particular district. The team supervisor shall arrange this accommodation with assistance from the state health supervisor. |
|
| iii) | The supervisors from NTI/any other collaborating agency shall make a planning visit well in advance of the initiation of the survey to make necessary arrangements including accommodation, identification of clusters, hiring of vehicles, storage o f tuberculin, eliciting necessary support from the local health authorities, etc. They shall also brief the DHO, DTO and other relevant officials about the survey during their visit. |
|
| iv) | The selection of clusters in each district shall be done by Stastitical Section of NTI. The list of selected clusters shall be provided to the Team Supervisor. | |
| v) | To ascertain the location of clusters, the planning team is to seek help from the District Health Authorities. The District Health Authorities shall be requested by NTI/collaborating agency to obtain a district census handbook from the local census office and also the census map of the district for easy location of the cluster. |
|
| vi) | The planning team shall visit the selected cluster and contact the village heads or some responsible person of the village and brief them about the purpose of the survey. The team shall elicit their cooperation for smooth conduct of the study and inform them about the date and time of the testing schedule. The planning sheet (NSS form-1) shall be filled up as per the columns given (self-explanatory). |
|
| vii) | Selection of starting point for registration: During the planning visit to the cluster, a rough map of the village and hamlet is to be drawn with the help of the village leaders. All the lanes of the main village and the hamlets will be numbered serially and it should be shown on the map. The numbering of lanes is to be done in a clockwise direction. |
|
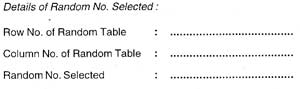
| A two-stage selection of the starting point will be done using random number table:- | ||
| - | In the first stage, it will be decided whether to start the survey from the main village or the hamlets. |
|
| - | In the second stage, the starting lane shall be selected. |
|
| - | The lane number selected shall be indicated on the planning sheet and the random number table to be maintained by the Team Supervisor. This information is to be passed on to the registration and testing team. |
|
| The first household at the north west end of the selected lane shall be the starting point for registration. The registration shall continue into the next higher serially numbered lanes. | ||
REGISTRATION
|
||
| i) | The registration team is to commence work from the first household selected earlier by the planning team. All the households in that lane are to be numbered serially, with a permanent marker, the start house being given the No.001. The households are numbered irrespective of the presence of eligible children for the survey. In case of more than one household in a single building, the households shall be numbered as for e.g., 001/A, 001/B, and so on. The registration team shall move to the adjacent household and so on till the last household of the lane. Thereafter, it shall move to the lane with the next higher serial numberand cover the households similarly. |
|
| ii) | Filling of NSS form 2 : The household number and the lane number will be entered in Column No.1 of NSS form-2 irrespective of the presence or absence of children aged 1-9 years. In each household, enquiry shall be made about the presence of children aged 1-9 years from the head of the family or some responsible person. In case, an eligible child belongs to the household, other details in the row shall be filled in. The particulars of another eligible child in the household shall be entered in the subsequent rows and so on. Nil entry shall be marked in case there is no child in this age group in the household. Care must be taken to estimate age as correctly as possible by talking to the parents/guardians relating with important events in the family and by personal assessment. In case of doubt verify from available documents. Only the children in this age group shall be registered and included in the study. The date of birth eligibility shall be pre-entered at the top of the form by the Supervisor. For e.g., if a cluster is being surveyed on 31.10.1999, all children born between 1.11.1989 and 31.10.1998 shall be registered. The name, age, sex, and the Child No. of the children available for testing is to be entered in the relevant columns of NSS form 2. |
|
| The registration team shall enquire about the residential status of each child in the household. Only permanent residents (children living in the cluster for more than 6 months) shall be registered and included in the study. |
||
| Each available child (permanent resident) shall be registered in the individual child card (NSS form-3). The child cards are issued only for the eligible children who are available at the time of registration or likely to be available in the day. The remarks column of NSS form 2 would contain information on the reasons of the absence of any child from the house. |
||
| iii) | The enumerator shall fill columns 1 to 8 of NSS form-3 at the time of registration as per the instructions given below: |
|
| iv) | The registration team is expected to visit adequate number of households in order to register 85 children. In the event of non-availability of 85 children in the entire cluster including hamlets, the registration is to continue in the geographically adjacent cluster on the same day till the requisite numbers are registered. On collecting sufficient number of children, one of the members of the census team shall guide the children to the testing centre and shall give the registration cards to the tester-secretary. |
|
v) |
At the time of registration, parents/guardians of each child shall be requested to see that the child remains available on the date of reading.
|
|
TUBERCULIN TESTING
|
||
(i) |
A central place under a shade shall be set
up for tuberculin testing. |
|
(ii) |
Written consent : At the testing
center, the procedure for the test, its side effects and the purpose
of the test shall be explained to the parent/guardian. The written
consent for participation in the survey shall be obtained in the
consent form designed by the Ethical Committee. Only in case of
non-availability of either of the parent at the time of survey,
the written consent shall be obtained from any responsible person
of that household. |
|
| (iii) | BCG scar examination : The child shall be examined by Tester-Secretary on the upper third of both the arms for presence/absence of BCG scar which is a pea-sized (2 to 3mm) hypo-pigmented shiny lesion raised above the skin and the finding shall be entered in the relevant box. | |
| (iv) | Injection of tuberculin : The child is to be injected intradermally on the mid volar aspect of the left forearm by the tuberculin tester, with 1TU of PPD RT23 with Tween 80 using a disposable 1 ml tuberculin syringe to which a needle of 26½ gauge and half an inch bevel is attached. The procedure should be in accordance to that outlined in the testing manual. Injection of each test shall be observed by the tester-secretary and the testing status shall be marked as mentioned below: |
|
| (v) | Testing status : S ( satisfactory): A satisfactory test should give rise to a hypo-pigmented weal about 5 to 6 mm above the skin level with pits and clear hair follicles. The tests should be strictly intradermal and without any leakage of tuberculin. U (unsatisfactory): The test is given subcutaneously / leakage of tuberculin / non-formation of the weal as described above. |
|
| (vi) | The columns 9 to 12 of NSS-3 shall be filled by the tester-secretary as per the instructions given below: |
|
| (vii) | At the end of the day, all forms and cards shall be checked once again by the testing team for correctness and completeness of all entries before leaving the place.
|
|
TUBERCULIN READING
|
||
| (i) | Reading shall normally be done after 3 days (72 hours) of testing but can be undertaken after 2 days (48 hours) or 4 days (96 hours) only in case of exigencies. In each cluster, a minimum of 2 rounds (house to house visits) shall be made in order to attain at least 80% reading coverage of the registered children on a single day. |
|
| (ii) | On the day of reading before proceeding to the field, the reader shall collect all the child cards (NSS-3), which are due for reading in that cluster. The reading team shall go house to house carrying with them the NSS forms 1 and 3 (filled earlier) for the tested children. After verifying the identity of the tested children, the reader is to locate the tested spot and by slowly palpating the edges of the reactions, the maximum transverse diameter of the induration is to be measured by a transparent mm scale after delineating the edges of the induration with a ball pen and dictate the reaction size to the reader-secretary. It may be noted that the reading is limited to induration only and transverse diameter to be measured is relative to the forearm. The induration may vary from a well-circumscribed density in the skin to a soft ill-defined swelling. In order to avoid the influence of inter-reader variation on survey results, the readings in a particular district shall be performed by the same trained reader. |
|
| (iii) | The reader-secretary shall fill columns 13 to 16 of NSS form 3 as per the instructions given below: |
|
| (iv) | All the children suspicious of suffering from tuberculosis shall be issued a referral slip (NSS form 4) with the advice to consult the medical officer incharge of the nearest Government Health Centre for further investigations and treatment if required. DHOs would be requested in advance to issue a circular to PHIs in this respect. All the cards and forms at the end of the day shall be re-examined for correctness and completeness. |
|
| (v) | The entries made in column 4, 7, 8, 9, 11, 12, 15 and 16 of NSS form 3 shall be transferred to NSS form 5 for duplicate entries by one of the Field Investigators. The Team Leader shall crosscheck the transferred entries. These duplicate entries are a safeguard against loss of data during transit.
|
|
TRANSPORT
|
||
The Team Leader with the support of local health personnel shall hire a suitable vehicle with a capacity of 10 for all the days of the field work. On any working day, the vehicle shall first drop the registration and testing teams, then the reading team and take the planning team to the planning village and follow the reverse order on return.
|
||
QUALITY CONTROL
|
||
| i) | The quality control of tuberculin testing shall be performed by direct observation of the tests given by field workers. The Supervisors from NTI and other Collaborating Institutions shall do this observation. The proportion of unsatisfactory tests shall not be allowed to exceed 2-3%. |
|
| ii) | For quality control of reading of the reactions, 5% of the reaction sizes shall be read independently by these Supervisors. Their readings are to be recorded in a separate sheet for ensuring internal quality control (NSS form 5). Corrective actions shall be undertaken as and when required. |
|
| iii) | The quality control of data entry shall be undertaken by scrutiny of the day's work including individual entries in child cards at the end of each day's work. |
|
| iv) | The data shall again be rechecked at NTI and in case of gross discrepancies from any clusters, appropriate number of new clusters shall be selected from that district to complete the required number of clusters from the district. |
|
|
||
MAINTENANCE OF COLD CHAIN
The tuberculin vials shall be transported by air from BCG Lab, Guindy, to the zonal headquarters for the survey, for e.g., NTI for south zone. Utmost care should be taken to maintain the temperature of the refrigerator between 4-8o C and tuberculin must not be allowed to freeze. The Team Leader would check the thermal regulator and thermometer as well as the reliability of electric current before placing the vials in the refrigerator. The vials will be transported to the survey districts in vaccine carriers. On reaching the district, the vials shall again be refrigerated at a proper facility either at district level or at PHC level wherever convenient and shall be carried to the field areas in vaccine carriers/ thermos flasks containing ice. Care will be taken not to expose the vials to sun light/heat and the testing centres shall be set up only at cool shaded places. The zonal centre shall obtain the supply of tuberculin from BCG Lab on a quarterly basis for each zone and the vials shall be consumed within the expiry date.
TUBERCULIN POTENCY AND SAFETY TESTS
The BCG Lab shall provide the information on the safety and the potency tests conducted for each batch of tuberculin. To avoid the influence of batch to batch variation, one single batch of tuberculin shall be used in a particular district. The batch number of tuberculin vials used in each district shall be recorded and maintained by the zonal centers.

 |




